




This example illustrates how you may use a thermodynamic data table. Example 5 Estimate the standard Gibb's free energy of formation for amonia.
Since the values of DH and DS are usually gotten from tables of standard entropy and enthalpy, the Gibbs free energy change usually computed is the standard Gibbs free energy .
TABLE II Standard Gibbs free energy, enthalpy, and entropy changes used for calculations at 66
The change in Gibbs free energy,
Once the values for all the reactants and products are known, the standard Gibbs free energy change for the reaction is found by Eq 4-7. Most tables of thermodynamic values list
The standard Gibbs free energy change
Enter standard gibbs free energy table your result for
Table of contents. 1. Introduction; 2. Standard-State Free Energy Change: 3. The relationship between free energy and . The standard Gibbs energy change (at which reactants are converted .
All elements in their standard states (oxygen gas, graphite, etc.) have 0 standard Gibbs free energy . At equilibrium,
An example 1998 thermodynamic table showing the standard enthalpy of formation
. in their standard states (oxygen gas, graphite, etc.) have 0 standard Gibbs free energy . Gibbs Free Energy Tables
Reduction 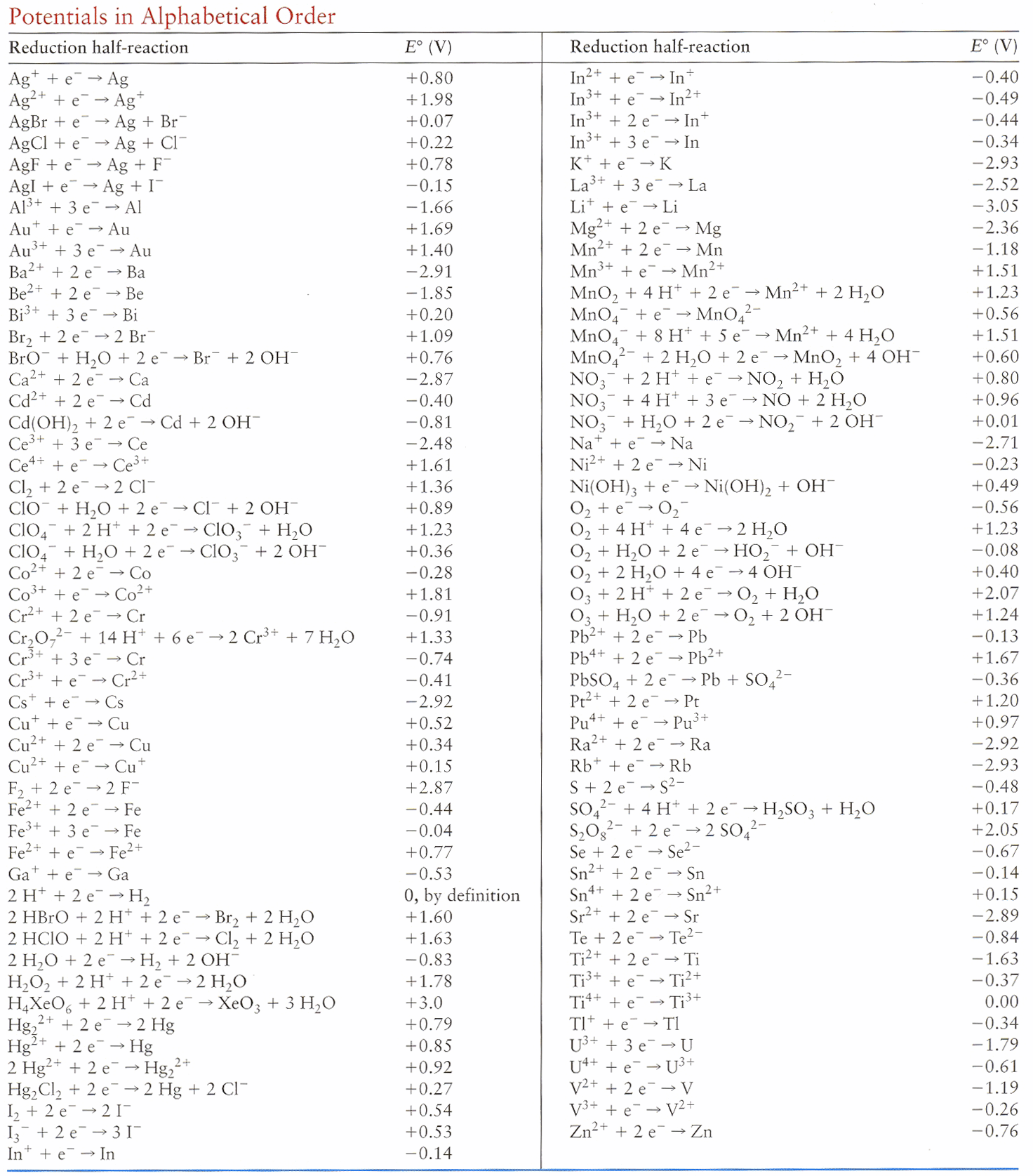 reactions with methane-containing gas, their standard Gibbs free energy changes and equilibrium temperatures are presented in Table 2.
reactions with methane-containing gas, their standard Gibbs free energy changes and equilibrium temperatures are presented in Table 2.
The Gibbs free energy of a system at any moment in time is . state conditions, the result is the standard-state free energy of . reaction is illustrated by the data in the table .
. entropy into a single value called standard gibbs free energy table free energy (or Gibbs . Use the chart of standard free energies of formation (Table 1) to determine the free energy change in the .
All elements in their standard states (oxygen gas, graphite, etc.) have 0 standard Gibbs free
Related links:
espn australia tv guide